The Relationship Between Composite Inflammatory Ratios and Complications of Massive Ischaemic Stroke
By Liqin Zheng, Zhijian LinAffiliations
doi: 10.29271/jcpsp.2024.04.434ABSTRACT
Objective: To investigate the relationship between complications of massive cerebral infarction (MCI) and composite inflammatory ratios.
Study Design: A case-control study.
Place and Duration of the Study: Department of Neurology, Affiliated Hospital of Putian University, Putian, China, from January 2019 to November 2021.
Methodology: Eighty-two patients with MCI underwent blood tests within 24 hours of admission. Complications such as cerebral herniation, haemorrhage transformation (HT), and stroke-associated pneumonia (SAP) were evaluated based on imaging examinations. The prognosis was assessed using the modified Rankin Scale score (mRS) at discharge.
Results: Among the 82 patients, the cerebral herniation group had higher levels of systemic immune inflammation index (SII) and neutrophil-to-lymphocyte ratio (NLR) compared to the non-cerebral herniation group. MCI patients who developed HT had higher levels of SII, NLR, mean platelet volume/platelet (MPV/PLT), and platelet-to-lymphocyte ratio (PLR). The SAP group had higher levels of MPV/PLT and NLR compared to the non-SAP group. The poor prognosis group had higher SII and NLR levels but a lower lymphocyte-to-monocyte ratio (LMR) compared to the good prognosis group.
Conclusion: NLR showed high accuracy in predicting complications and the short-term prognosis of MCI. SII was linked to cerebral herniation, HT, and the short-term prognosis of MCI. MPV/PLT was found to be related to SAP and HT caused by MCI. LMR may act as a protective factor for the short-term prognosis of MCI.
Key Words: Massive cerebral infarction, Neutrophil-to-lymphocyte ratio, Systemic immune inflammation index, Prognosis.
INTRODUCTION
Massive cerebral infarction (MCI) is a severe type of stroke caused by the occlusion of the proximal middle cerebral artery (MCA) or the internal carotid artery (ICA) on one side. It accounts for 10 to 20% of acute ischaemic strokes (AIS) and has a high mortality rate.1 Within 3-5 days, approximately half of MCI cases progress to cerebral herniation, which is the leading cause of death in MCI.2 Haemorrhage transformation (HT) and stroke-associated pneumonia (SAP) are also serious complications of MCI.3 However, the factors that affect the development of these complications and the prognosis of MCI remain uncertain. Currently, the diagnosis of MCI complications relies mainly on imaging examinations, which are not quick to detect. Therefore, it is necessary to obtain rapid laboratory indicators that can predict the complications of MCI and improve prognosis.
Numerous studies have confirmed that inflammation plays a key role in the pathological process and prognosis of AIS. Additionally, studies have suggested that the neutrophil-to-lymphocyte ratio (NLR) and systemic immune inflammation index (SII) have precise predictive value for AIS.4-6 However, there are only a few studies that have focused on the relationship between composite inflammatory ratios and complications of MCI, as well as the prognosis of MCI.7,8 Therefore, this study aimed to investigate the composite inflammatory ratios that predict complications and the short-term prognosis of patients with MCI.
METHODOLOGY
MCI patients were consecutively recruited between January 2019 and November 2021, with a total of 82 participants. The study received approval from the ethics committee of the Affiliated Hospital of Putian University (Approval Number: 202202).
The diagnosis of MCI was conducted following the guidelines for the management of large hemispheric infarction. The diagnosis of SAP was based on the 2015 diagnosis consensus.9 Diagnosis of cerebral herniation and HT were confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). Short-term prognosis of MCI was evaluated based on the modified Rankin Scale (mRS) score at discharge.
Inclusion criteria were participants aged 18 years or older with unilateral MCI that affects at least two-thirds of the MCA territory. Exclusion criteria were incomplete data of blood cell counts (n = 20), active infection within 2 weeks (n = 10), onset time >48 hours (n = 6), history of cancer or haematologic disease (n = 7), severe hepatic or renal diseases (n = 8), and mRS >2 before onset (n = 2). In the end, a total of 82 patients were enrolled in the study.
The authors collected clinical data from 82 consecutive patients who were diagnosed with MCI through the case system. The following data were analysed: Age, gender, vascular risk factors (hypertension, diabetes mellitus (DM), atrial fibrillation (AF), and previous stroke), clinical assessment (systolic blood pressure (SBP), diastolic blood pressure (DBP) at admission, the score of the National Institute of Health Stroke Scale (NIHSS) at admission, and the score of the mRS at discharge), vascular reperfusion therapy (intravenous thrombolysis or arterial thrombectomy), and laboratory examinations at admission.
All blood samples were collected within 24 hours of admission. NLR was calculated as neutrophil count divided by lymphocyte count. The platelet-to-lymphocyte ratio (PLR) was calculated as platelet count divided by lymphocyte count. The lymphocyte-to-monocyte ratio (LMR) was calculated as lymphocyte count divided by monocyte count. The mean platelet volume (MPV) / platelet (PLT) was calculated as MPV divided by PLT. SII was calculated using the formula: SII = PLT * neutrophil count / lymphocyte count.10
Statistical analysis was conducted using SPSS software (IBM SPSS 25.0). The Shapiro-Wilk method was employed to test the distribution of quantitative data. Normally distributed quantitative data were compared using the independent sample Student's t-test and presented as mean ± standard deviation (SD), while skewed distribution data were compared using the Mann-Whitney U-test and presented as median (25th percentile, 75th percentile). The Chi-square test was used to compare differences in qualitative data, which were presented as numbers (percentages). A two-tailed value of p <0.05 was considered statistically significant. Receiver Operator Characteristic (ROC) curves were utilised to assess the predictive value of peripheral inflammation indicators for the complications and prognosis of MCI. When p-values were ≤0.05, the difference in the area under the ROC curves (AUCs) was deemed significant.
RESULTS
Patients with cerebral herniation had higher NIHSS scores and blood pressure at admission. The levels of SII in the cerebral herniation group were higher than those in the non-cerebral herniation group, as well as NLR. The optimal cut-off value of NLR and SII for cerebral herniation caused by MCI were 9.5164 (sensitivity 85.7%, specificity 69.3%) and 1798 (sensitivity 85.7%, specificity 68%, Figure 1). The data of the MCI patients are presented in Table I.
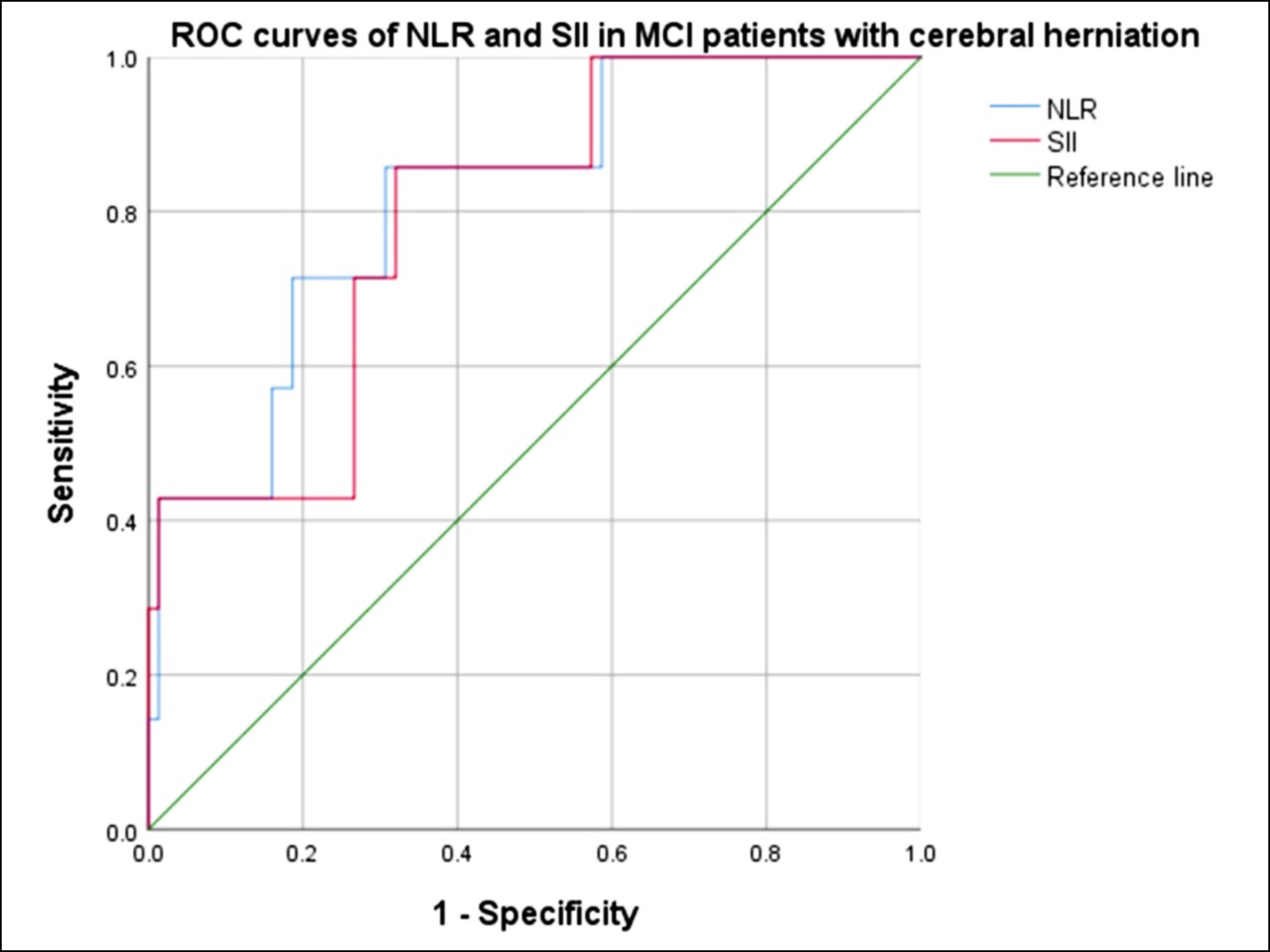 Figure 1: ROC curves of NLR and SII in patients with cerebral herniation. The AUC of NLR was 0.819 [95% confidence interval (CI), 0.664-0.974, p = 0.005]. The AUC of SII to discriminate cerebral herniation was 0.794 (95% CI, 0.636-0.953, p = 0.01).
Figure 1: ROC curves of NLR and SII in patients with cerebral herniation. The AUC of NLR was 0.819 [95% confidence interval (CI), 0.664-0.974, p = 0.005]. The AUC of SII to discriminate cerebral herniation was 0.794 (95% CI, 0.636-0.953, p = 0.01).
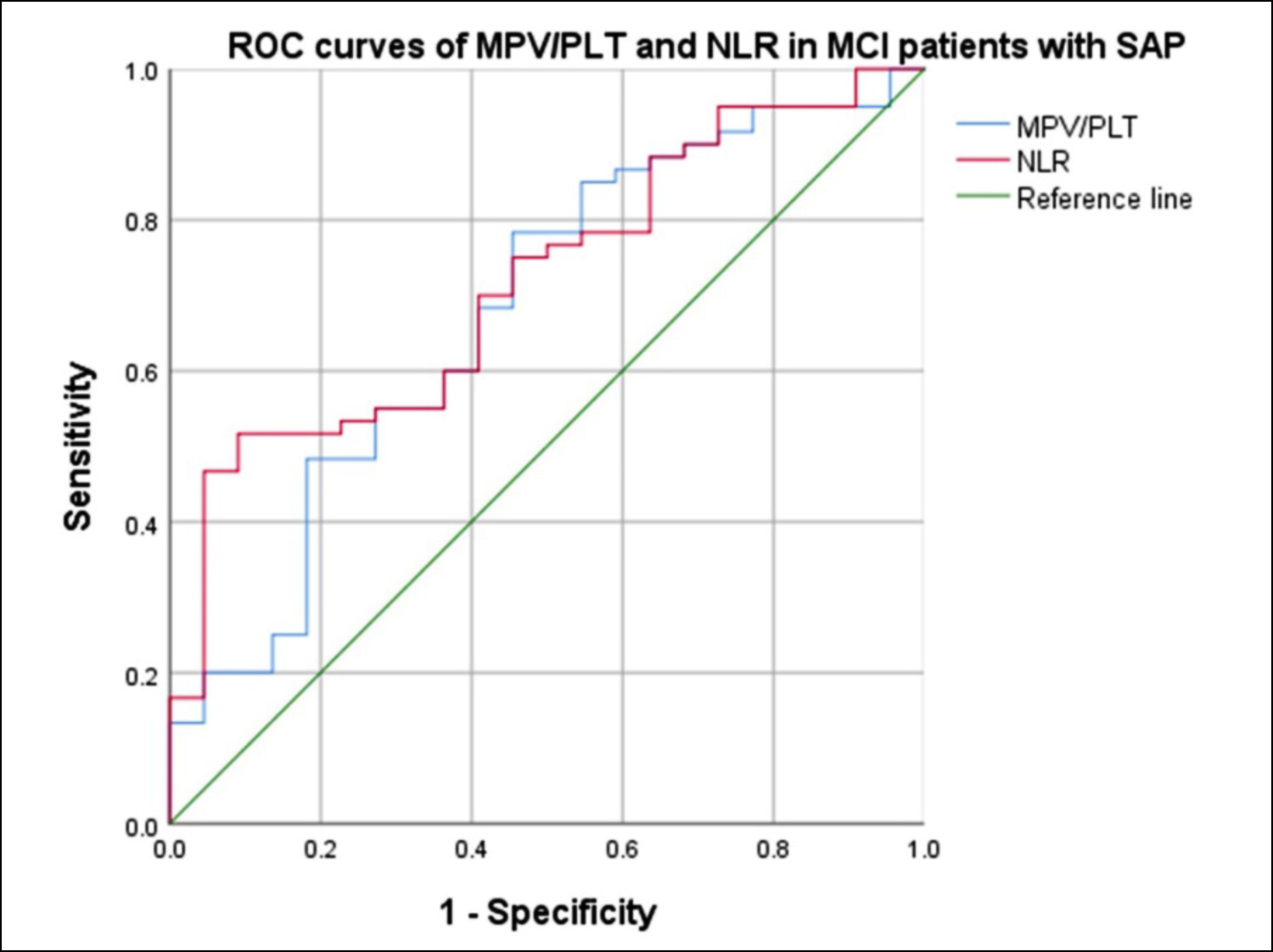 Figure 2: ROC curves of MPV/PLT and NLR in MCI patients with SAP. The AUC of NLR was 0.717 (95% CI, 0.600-0.835, p = 0.003), which was larger than that of the MPV/PLT (0.678, 95% CI, 0.545-0.812, p = 0.014), suggesting that the NLR was a superior biomarker for SAP caused by MCI.
Figure 2: ROC curves of MPV/PLT and NLR in MCI patients with SAP. The AUC of NLR was 0.717 (95% CI, 0.600-0.835, p = 0.003), which was larger than that of the MPV/PLT (0.678, 95% CI, 0.545-0.812, p = 0.014), suggesting that the NLR was a superior biomarker for SAP caused by MCI.
Significant differences were observed between the non-HT group and the HT group in various factors, including the history of AF,11 NIHSS score, vascular reperfusion therapy, and lesion location. Patients in the HT group exhibited elevated levels in several laboratory examinations, such as SII, MPV/PLT, NLR, and PLR. However, it was noted that only the AUC of NLR was larger than 0.7, indicating that NLR is a precise index for predicting HT caused by MCI. Further details can be found in Table II.
The patients in the SAP group had higher levels of MPV/PLT and NLR. The optimal cut-off value for NLR as a risk factor for predicting SAP caused by MCI was 8.69 (sensitivity 51.7%, specificity 90.9%), while the MPV/PLT was 0.04 (sensitivity 78.3%, specificity 54.5% Figure 2).
As shown in Table III, the group with a poor prognosis had a higher NIHSS score and a higher rate of cerebral herniation. It was observed that the group with a poor prognosis also had higher SII and NLR values.
Table I: Demographical characteristics and clinical data of the subgroup.
|
Variables |
Non-cerebral herniation group (n=75) |
Cerebral herniation group (n=7) |
p-value |
Non-HT group (n=46) |
HT group (n=36) |
p-value |
Non-SAP group (n=22) |
SAP group (n=60) |
p-value |
|
Demographics |
|||||||||
|
Age, years |
69(62, 80) |
68(52, 70) |
0.421 |
67.78 ± 12.93 |
70.19 ± 10.55 |
0.367 |
66.5(59.0, 73.8) |
70.0(63.0, 83.8) |
0.187 |
|
Male, n (%) |
48(64.0) |
4(57.1) |
>0.99 |
32(69.6) |
20(55.6) |
0.191 |
16(72.7) |
36(60.0) |
0.289 |
|
Vascular risk factors, n(%) |
|||||||||
|
Hypertension n (%) |
35(46.7) |
5(71.4) |
0.391 |
23(50.0) |
17(47.2) |
0.803 |
12(54.5) |
28(46.7) |
0.527 |
|
Diabetes mellitus n (%) |
16(21.3) |
3(42.9) |
0.411 |
12(26.1) |
7(19.4) |
0.479 |
5(22.7) |
14(23.3) |
0.954 |
|
Atrial fibrillation n (%) |
38(50.7) |
3(42.9) |
1.000 |
16(34.8) |
25(69.4) |
0.002* |
7(31.8) |
34(56.7) |
0.046* |
|
Previous stroke n (%) |
4(5.3) |
2(28.6) |
0.134 |
5(10.9) |
1(2.8) |
0.332 |
3(13.6) |
3(5.0) |
0.394 |
|
Clinical assessment |
|||||||||
|
NIHSS score at admission |
11(7, 18) |
20(15, 23) |
0.030* |
10(4, 13) |
18(13, 22) |
<0.001* |
7.00(3.00, 10.25) |
15.00(10.00, 20.00) |
<0.001* |
|
SBP, mmHg |
142.55 ± 24.13 |
170.57 ± 30.61 |
0.005* |
144.72 ± 27.33 |
145.22 ± 23.98 |
0.930 |
140.09 ± 19.92 |
146.72 ± 27.52 |
0.305 |
|
DBP, mmHg |
81.48 ± 15.16 |
100.14 ± 19.61 |
0.003* |
81.65 ± 13.68 |
84.89 ± 19.21 |
0.376 |
81.95 ± 11.82 |
83.48 ± 17.74 |
0.709 |
|
Vascular reperfusion therapy |
|||||||||
|
Intravenous thrombolysis, n (%) |
12(16.0) |
2(28.6) |
0.749 |
3(6.5) |
11(30.6) |
0.01* |
1(4.5) |
13(21.7) |
0.135 |
|
Arterial thrombectomy, n (%) |
27(36.0) |
1(14.3) |
0.413 |
7(15.2) |
21(58.3) |
<0.001* |
3(13.6) |
25(41.7) |
0.035* |
|
Laboratory data |
|||||||||
|
SII |
1336.41 (833.34, 2242.55) |
2184.13 (1849.47, 4724.67) |
0.010* |
1210.33 (733.22, 2024.29) |
1798.00 (1142.37, 2544.97) |
0.014* |
1160.74 (718.85, 1707.45) |
1703.02 (951.06, 2514.72) |
0.052 |
|
MPV/PLT |
0.051(0.037, 0.067) |
0.059(0.055, 0.060) |
0.242 |
0.05 ± 0.029 |
0.06 ± 0.02 |
0.023* |
0.043(0.031, 0.058) |
0.056(0.044, 0.067) |
0.014* |
|
NLR |
6.48(4.50, 10.34) |
11.09(9.58, 27.63) |
0.005* |
5.49,(3.30, 8.62) |
9.89(5.93, 11.66) |
<0.001* |
5.08(2.83, 8.17) |
8.93(5.14, 11.07) |
0.003* |
|
PLR |
187.74 (131.58, 232.95) |
214.13 (172.27, 316.67) |
0.198 |
165.95 (123.14, 228.98) |
210.11 (173.46, 277.20) |
0.028* |
161.78 (131.79, 238.62) |
188.66 (132.26, 246.67) |
0.402 |
|
LMR |
2.25(1.59, 3.77) |
1.31(0.69, 2.83) |
0.077 |
2.30(1.57, 4.06) |
2.04(1.52, 3.29) |
0.353 |
2.43(1.52, 4.36) |
2.18(1.57, 3.36) |
0.336 |
|
Statistical test applied: Independent sample Student’s t-test, Mann-Whitney U-test and Chi-square test. p <0.05 is statistically significant. * Statistically significant. MCI: Massive ischaemic stroke, HT: Haemorrhage transformation, NIHSS: National Institute of Health Stroke Scale, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, SII: Systemic immune inflammation index, MPV/PLT: Mean platelet volume/platelet, NLR: Neutrophil-to-lymphocyte ratio, PLR: Platelet-to-lymphocyte ratio, LMR: Lymphocyte-to-monocyte ratio. |
|||||||||
Table II: Prediction of HT using NLR, SII, MPV/PLT, and PLR as risk factors for MCI patients.
|
Factors |
AUC |
95%CI |
p-value |
Cut-off point |
Sensitivity |
Specificity |
|
NLR |
0.739 |
0.632-0.846 |
<0.001 |
9.5164 |
58.3% |
82.6% |
|
SII |
0.659 |
0.541-0.776 |
0.014 |
1721.7914 |
58.3% |
71.7% |
|
MPV/PLT |
0.654 |
0.533-0.774 |
0.017 |
0.0545 |
63.9% |
65.2% |
|
PLR |
0.642 |
0.521-0.762 |
0.028 |
176.9117 |
75.0% |
58.7% |
|
HT: Haemorrhage transformation, NLR: Neutrophil-to-lymphocyte ratio, SII: Systemic immune inflammation index, MPV/PLT: Mean platelet volume/platelet, PLR: Platelet-to-lymphocyte ratio, MCI: Massive ischaemic stroke. |
||||||
|
Variables |
Poor prognosis group (mRs ≥5) (n = 13) |
Good prognosis group (mRs ≤4) (n = 69) |
p-value |
|
Demographics |
- |
- |
- |
|
Age, years |
70 (57.5, 76) |
68 (61.5, 80.5) |
0.849 |
|
Male, n (%) |
8 (61.5) |
44 (63.8) |
0.878 |
|
Clinical assessment |
- |
- |
- |
|
NIHSS, score |
19.0 (15.5, 29.0) |
11.0 (6.5, 17.5) |
0.001* |
|
SBP, mmHg |
151.85 ± 34.70 |
143.64 ± 23.81 |
0.295 |
|
DBP, mmHg |
90.92 ± 21.96 |
81.59 ± 14.76 |
0.058 |
|
Vascular reperfusion therapy |
- |
- |
- |
|
Intravenous thrombolysis, n (%) |
1 (7.7) |
13 (18.8) |
0.563 |
|
Arterial thrombectomy, n (%) |
2 (15.4) |
26 (37.7) |
0.216 |
|
Complication, n (%) |
- |
- |
- |
|
Cerebral herniation |
6 (46.2) |
1 (1.4) |
<0.001* |
|
HT |
8 (61.5) |
28 (40.6)) |
0.162 |
|
SAP |
12 (92.3) |
48 (69.6) |
0.175 |
|
Laboratory data |
- |
- |
- |
|
MPV, f L |
10.50 (9.10, 11.20) |
10.30 (9.35, 10.90) |
0.717 |
|
SII |
2101.00 (1296.73, 3746.19) |
1293.71 (831.21, 2197.86) |
0.012* |
|
MPV/PLT |
0.059 (0.048, 0.071) |
0.051 (0.037, 0.067) |
0.272 |
|
NLR |
11.00 (8.17, 18.53) |
6.09 (4.47, 10.12) |
0.006* |
|
PLR |
188.57 (174.52, 241.48) |
187.74 (130.15, 245.35) |
0.384 |
|
LMR |
1.37 (0.76, 2.74) |
2.26 (1.73, 3.94) |
0.013* |
|
Statistical test applied: Independent sample Student’s t-test, Mann-Whitney U-test and Chi-square test. p <0.05 is statistically significant. * Statistically significant. MCI: Massive ischaemic stroke, NIHSS: National Institute of Health Stroke Scale, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, HT: Haemorrhage transformation, SAP: Stroke-associated pneumonia, MPV: Mean platelet volume, SII: Systemic immune inflammation index, MPV/PLT: Mean platelet volume/platelet, NLR: Neutrophil-to-lymphocyte ratio, PLR: Platelet-to lymphocyte ratio, LMR: Lymphocyte-to-monocyte ratio. |
|||
Specifically, the group with a poor prognosis had a lower LMR. ROC curves were used to assess the prognostic value of NLR, SII, and LMR. The AUCs were 0.740 [95% CI, (0.594-0.887), p = 0.006] for NLR and 0.721 [95% CI, (0.561-0.881), p = 0.012] for SII. The optimal cut-off values to distinguish patients with poor outcomes were 7.93 for NLR (sensitivity 84.6%, specificity 60.9%) and 1192 for SII (sensitivity 92.3%, specificity 44.9%). The AUC of LMR as a protective factor was 0.719 [95% CI, (0.536-0.902), sensitivity 92.8%, specificity 53.8%, p = 0.013, cut-off point =1.37].
DISCUSSION
This research found that the NLR was highly accurate in predicting complications of MCI and short-term prognosis. SII was linked to cerebral herniation, HT, and the short-term prognosis of MCI. MPV/PLT may be related to SAP and HT caused by MCI. Finally, LMR may be a protective factor for the short-term prognosis of MCI.
These findings emphasise the critical importance of NLR in MCI. The inflammatory response has been implicated in the pathogenesis of stroke. When AIS occurs, it triggers the recruitment of multiple immune cells into the brain to initiate an inflammatory response. Neutrophils, which can be detected within 15-60 minutes following AIS, are recruited to the ischaemic tissue and induce the production of free oxygen radicals.12 Additionally, neutrophils can release molecules that interact with platelets and contribute to the formation of neutrophil extracellular traps, thereby exacerbating thrombosis.13 Furthermore, neutrophils, acting as a source of matrix metalloproteinase-9, can lead to HT and symptomatic deterioration. Several studies have shown that lymphocytes act as protective factors against AIS.14,15 Regulatory T cells interact with other cells and produce anti-inflammatory cytokines to modulate various immune pathways and maintain immune homeostasis.16 Regulatory B cells can limit inflammation in the central nervous system of stroke mice and reduce neurological deficits.17 Previous studies have revealed a positive association between NLR and the development of HT and poor prognosis following AIS.18 Supporting the author's findings, a study demonstrated that NLR can serve as a risk predictor for cerebral herniation, neurological recovery, and prognosis in MCI patients.7
Compared to NLR, SII emphasises the importance of PLT. PLT can activate the immune response to ischaemic brain tissue by interacting with leukocytes and plays a significant role in initiating and promoting the development of atherosclerotic lesions.19,20 Elevated SII has been reported to predict the development of HT after anterior circulation infarction and the severity of AIS, as well as the prognosis of AIS treated with intravenous thrombolysis.21,22 This study demonstrated that SII could predict the development of HT and the short-term prognosis of MCI, not just AIS. Most notably, this is the first study to indicate the relationship between SII and cerebral herniation caused by MCI.
This research proposed that MPV/PLT is related to SAP and HT caused by MCI for the first time. A previous study indicated that high MPV is an independent predictor of poor prognosis for AIS.23 However, in line with this study, a recent study found no correlation between MPV and prognosis of MCI.24
Furthermore, it was observed that LMR was a positive factor for the short-term prognosis of MCI. Monocytes release matrix metalloproteinase-9, which causes HT and symptomatic deterioration. In contrast to this study, previous studies showed that LMR was correlated with poor outcomes in AIS with intravenous thrombolysis and HT caused by AIS.25
This research has the following advantages: Firstly, it is a relatively comprehensive study that examines the association between multiple composite inflammatory ratios and MCI. Secondly, the study had several significant findings of SII being significantly linked to cerebral herniation caused by MCI and MPV/PLT relation to SAP and HT. It was observed that LMR was a positive factor for the short-term prognosis of MCI. Finally, compared to the most previous studies that recruited patients within 7 days of symptom onset, this study recruited patients within 48 hours of symptom onset to obtain inflammatory markers in the early stage.
There are several limitations to this study. Firstly, it was a single-centric retrospective study, and a multi-centric prospective study is required. Due to the limited sample size, this study did not perform logistic regression analysis on significant confounding factors such as age, NIHSS score, atrial fibrillation, hypertension, diabetes, and history of previous stroke. Secondly, the dynamic changes of inflammatory markers were not collected to investigate the relationship between immune cells and MCI. Finally, this study only investigated the contribution of the peripheral immune system to MCI. It is necessary to conduct a study focusing on the actual number of immune cells within the brain parenchyma in the future.
CONCLUSION
NLR had a high predictive value for complications and short-term prognosis of MCI. Apart from SAP, SII can predict cerebral herniation, HT, and short-term prognosis of MCI. It has been suggested that MPV/PLT is associated with SAP and HT resulting from MCI. Additionally, LMR may be a positive factor for the short-term prognosis of MCI. Further research is needed to validate these findings regarding MCI.
ETHICAL APPROVAL:
The study received approval from the ethics committee of the Affiliated Hospital of Putian University (Approval Number: 202202).
PATIENTS’ CONSENT:
The consent of the patients was taken prior to the initation of this study.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
LZ: Conceived, designed experiments, data collection, and follow-up.
LZ; ZL: Manuscript writing and modification.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Wijman CA. Editorial comment--Can we predict massive space-occupying edema in large hemispheric infarctions? Stroke 2003; 34(8):1899-900. doi: 10.1161/01.Str.00000 81986.02572.D5.
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol 1996; 53(4):309-15. doi: 10.1001/archneur.1996.005500 40037012.
- Brooks SD, Spears C, Cummings C, VanGilder RL, Stinehart KR, Gutmann L, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg 2014; 6(8):578-83. doi: 10.1136/neurintsurg-2013-010780.
- Becker KJ, Buckwalter M. Stroke, inflammation and the immune response: Dawn of a new era. Neurotherapeutics 2016; 13(4):659-60. doi: 10.1007/s13311-016-0478-7.
- Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 2016; 15(8):869-81. doi: 10.1016/s1474-4422(16)00114-9.
- Shichita T, Ito M, Morita R, Komai K, Noguchi Y, Ooboshi H, et al. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat Med 2017; 23(6):723-32. doi: 10.1038/ nm.4312.
- Huang Y, Li F, Chen Z, Chen W, Fan L, Zheng Y, et al. Predictive value of degranulating factors of neutrophils in massive cerebral infarction. Cell Transplant 2021; 30: 9636897211004089. doi: 10.1177/09636897211004089.
- Sun W, Li G, Liu Z, Miao J, Yang Z, Zhou Q, et al. A nomogram for predicting the in-hospital mortality after large hemispheric infarction. BMC Neurol 2019; 19(1):347. doi: 10.1186/s12883-019-1571-4.
- Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: Recommendations from the pneumonia in stroke consensus group. Stroke 2015; 46(8):2335-40. doi: 10.1161/strokeaha.115.009617.
- Zhu Z, Cong X, Li R, Yin X, Li C, Xue Y. Preoperative Systemic Immune-Inflammation Index (SII) for Predicting the Survival of Patients with Stage I-III Gastric Cancer with a Signet-Ring Cell (SRC) Component. Biomed Res Int 2020; 2020:5038217. doi: 10.1155/2020/5038217.
- Altuntas E, Karadeniz FO, Cetin S, Demir S. Relationship between atrial fibrillation and controlling nutritional status score in acute ischemic stroke patients. J Coll Physicians Surg Pak 2023; 33(2):165-9. doi: 10.29271/jcpsp.2023. 02.165.
- Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypo-thermia. J Neuroinflammation 2010; 7:74. doi: 10. 1186/ 1742-2094-7-74.
- Ruhnau J, Schulze J, Dressel A, Vogelgesang A. Thrombosis, neuroinflammation, and poststroke infection: The multifaceted role of neutrophils in stroke. J Immunol Res 2017; 2017:5140679. doi: 10.1155/2017/5140679.
- İdil Soylu A, Arıkan Cortcu S, Uzunkaya F, Atalay YO, Bekçi T, Güngör L, et al. The correlation of the platelet-to-lymphocyte ratio with the severity of stenosis and stroke in patients with carotid arterial disease. Vascular 2017; 25(3):299-306. doi: 10.1177/1708538116673770.
- Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol 2011; 10(5):471-80. doi: 10.1016/S1474-4422(11)70066-7.
- Bonaventura A, Liberale L, Vecchié A, Casula M, Carbone F, Dallegri F, et al. Update on inflammatory biomarkers and treatments in ischemic stroke. Int J Mol Sci 2016; 17(12). doi: 10.3390/ijms17121967.
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, et al. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci 2011; 31(23):8556-63. doi: 10.1523/jneurosci. 1623-11.2011.
- Yang Y, Han Y, Sun W, Zhang Y. Increased systemic immune-inflammation index predicts hemorrhagic transformation in anterior circulation acute ischemic stroke due to large-artery atherosclerotic. Int J Neurosci 2023; 133(6):629-35. doi: 10.1080/00207454.2021.1953021.
- Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res 2009; 104(3):346-54. doi: 10.1161/circresaha.108.185785.
- Lindemann S, Krämer B, Seizer P, Gawaz M. Platelets, inflammation and atherosclerosis. J Thromb Haemost 2007; 5(Suppl 1):203-11. doi: 10.1111/j.1538-7836. 2007.02517.x.
- Li LH, Chen CT, Chang YC, Chen YJ, Lee IH, How CK. Prognostic role of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index in acute ischemic stroke: A STROBE-compliant retrospective study. Medicine (Baltimore) 2021; 100(25): e26354. doi: 10.1097/MD.0000000000026354.
- Weng Y, Zeng T, Huang H, Ren J, Wang J, Yang C, et al. Systemic immune-inflammation index predicts 3-month functional outcome in acute ischemic stroke patients treated with intravenous thrombolysis. Clin Interv Aging 2021; 16:877-86. doi: 10.2147/CIA.S311047.
- Yao Y, Cao X, Zou R, Wen H, Zhang S, Xu H, et al. Study on the baseline factors and platelet indices that predict outcome of acute ischemic stroke patients after thrombolytic therapy. Cerebrovasc Dis 2022; 51(3):357-64. doi: 10. 1159/000519705.
- Chen Z, He Y, Su Y, Sun Y, Zhang Y, Chen H. Association of inflammatory and platelet volume markers with clinical outcome in patients with anterior circulation ischaemic stroke after endovascular thrombectomy. Neurol Res 2021; 43(6):503-10. doi: 10.1080/01616412.2020.1870359.
- Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation 2021; 18(1): 51. doi: 10.1186/s12974-021-02090-6.